An overview of oxygen scavenging packaging and applications

By Joanne Kaufman, Amanda LaCoste, James Schulok, Elia Shehady & Keith ‘Kit' Yam
Active packaging is defined as a packaging structure or process that responds to changes in its surrounding environment. In order to ensure safety and stability in our food supply — from harvest to consumption — the control of O2 degradation reactions in food products is paramount. Improvements in processing assist in this endeavor, but through the use of active packaging and, specifically, oxygen scavengers, oxygen's negative effects can be minimized. New, emerging technologies in oxygen scavenging such as sachets, extruded scavenging films, scavenging bottle closures and enzymatic O2 scavengers have improved the safety and quality of our food supply. This paper discusses the importance of oxygen scavenging, the benefits it provides to the food industry and the safety factors and risks involved.
Contents
Oxygen scavenging sachets
Oxygen scavengers in packaging materials
Polymers in the film
Addition of enzymes
The benefits of oxygen scavenging
Potential risks and/or disadvantages
Conclusion
Though oxygen is a key element in supporting life, when it comes to food shelf life, quality and, in some cases, safety, it can become an adversary. The element oxygen is essential to the biological functions in plants and animals involving respiration and photosynthesis. However in foods it causes the degradative oxidation reactions that result in off-flavors, odors and sometimes-harmful compounds.
Numerous research studies have been conducted to help develop packaging and processes that extend the shelf life of foods subject to oxidation reactions as well to prevent harmful compounds from being formed either from chemical reactions or microbial proliferation. Oxygen management is of particular importance in foods possessing high water activity (aw) and the ability to support microbial growth, as in the case of fresh produce and meat products.
One of the most effective methods of oxygen management in packaging and packaging process applications is the use of "active packaging." Active packaging refers to packaging and packaging systems that respond to changes in the surrounding environment.
More specifically, "oxygen scavengers" refers to a category within active packaging. Oxygen scavengers are packaging films or devices that chemically react with oxygen to remove it from the packaging environment. This, in turn, protects the product from degradative oxidation reactions. Oxygen scavengers exist in many forms, including sachets, films and enzymes, all of which can be used in food systems and function in a variety of ways.
Oxygen scavenging sachets
Oxygen scavenging sachets are packets of various sizes that are contained within a food package to absorb oxygen. Though primarily used in Asia, they are starting to gain acceptance in the United States. The Mitsubishi Gas Chemical Co. represents more than 70% of all sachet sales (Rooney, Chapter 6, 1995). Primarily used for shelf-life extension, sachets are highly effective and can reduce oxygen levels in a package to as low as 100 ppm ( Harima, 1990).

Any substance that reacts with oxygen can be considered an oxygen scavenger. However, when dealing with food, oxygen scavenging sachets must be safe, easily handled, compact in size and must not produce toxic substances or offensive odors/gases. They also must absorb a large amount of oxygen, have an appropriate oxygen absorption speed and be economically priced (Harima, 1990).
The two most common substances used in sachets are iron powder and ascorbic acid. An example of the oxidation reaction that takes place with the iron oxide is shown in Figure 1.
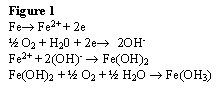
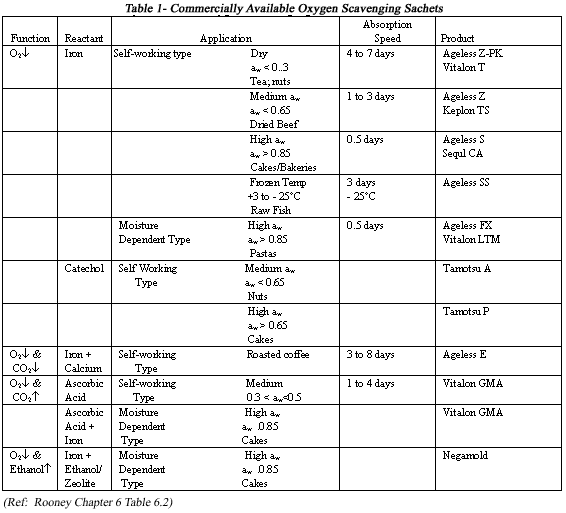
There are many commercially available oxygen scavenger sachets. Different sachets are used for different food formulations depending on aw and other intrinsic factors. A list of sachets for various conditions is provided in Table 1.
Oxygen scavengers are very reactive and will start to work once exposed to air. Therefore, they must be handled and packaged in such a way as to minimize exposure to the environment.
Sachets usually start to react with oxygen depending on the aw of the product, the storage temperature, the initial amount of O2 present in the headspace as well as dissolved in the food, and the O2 permeability of the package. Commercially available in a variety of different types, sachets are classified by their overall effectiveness to absorb oxygen. The three classes are as follows: immediate type (0.5 - 1 day); general type (1-4 days); and slow type (4-6 days) (Harima, 1990).
The choice of sachet to use in a given application depends on the intrinsic factors (aw, pH, etc.) of the food involved. For example, a moist food that could cause bacterial or mold growth would typically require a very fast oxygen-absorbing sachet, such as Mitsubishi's Ageless FX, while a dry food could use a less expensive, slower oxygen absorber like Ageless Z-PK.
Oxygen sachets are also used in conjunction with other sachets to improve overall product quality. These sachets include moisture absorbers and carbon dioxide absorbers and are mainly used in roasted coffee.
Oxygen scavengers in packaging materials
In addition to sachets, oxygen-scavenging compounds can be incorporated directly into the packaging material itself. These materials include flexible films, rigid plastics ( blow-molded or injection-molded polymers) and liners in closures.
The oxygen-scavenging mechanisms are very similar to those of the sachets. For example, Oxyguard from Toyo Seikan Group, a scavenger that uses an iron salt-based additive which absorbs oxygen, is in the form of a flexible or rigid plastic (Gabriele, 1999). The mechanism follows the reaction shown in Figure 1 above.
Oxygen-scavenging components added to the film would include an oxidizable metal, oxidation promoters and fillers. All of these components are usually food-contact approved compounds. They would be directly added to the resin or be a separate layer laminated or co-extruded with the other layers (Graff, 1998). Typically this oxygen-scavenging mechanism is moisture-activated.
For dry food products, a different approach must be taken due to their low aw. Initiating the reaction can be done by incorporating photon dyes into the film. The photosensitive dyes will increase the reaction of the oxygen with iron compounds. This was a result of the discovery that iron will not react quickly enough with ground state oxygen to successfully extend shelf life. This exposure will catalyze the reaction to scavenge the free oxygen in the headspace of the package. The film is illuminated with UV light that will excite the dye molecules, which sensitize oxygen molecules that are diffused into the film, changing them to the singlet state. The singlet oxygen molecules react with acceptor molecules and are consumed (Nielsen, 1997).
The basic reaction for the use of photosensitive dyes to increase reaction rate of iron-based oxygen absorbers is shown in Figure 2 (Vermeiren, 1999).
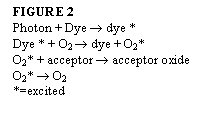
Polymers in the film
An alternate approach to metal-based oxygen-scavenging films is to use unique polymers to absorb headspace oxygen in packages. Polymers already have the capacity to react with oxygen (Thomas, 1998). However, by creating specific polymers to react with oxygen, the headspace oxygen will be reduced without the use of additives.
Specifically, Cryovac has developed its OS1000, which is a clear lidstock with a proprietary oxygen-scavenging layer coextruded in the film. This layer consists of an oxidizable polymer, a photoinitiator and a catalyst. The oxidizable polymer will react and bind with the oxygen. The photoinitiator will absorb the UV light and provides the energy to initiate the reaction (Thomas, 1998). The catalyst will reduce the activation energy for the overall reaction to occur.

Another example is by CMB Foodcan, where 7% nylon is blended with PET, and Cobalt stearate is added as a catalyst for blowmolded bottles (Nielsen, 1997).
A study conducted to evaluate the effectiveness of oxygen-scavenging films compared Mitsubishi's Ageless sachets and Cryovac's OS1000 on the color degradation of cured meats. The study demonstrated that both the sachet and oxygen-scavenging film effectively reduced color degradation by reducing the headspace oxygen below 0.5%. This was demonstrated with pepperoni and bologna. Also, OS1000 was shown to reduce the headspace oxygen level to below 0.1% to effectively retard the growth of mold and yeast in fresh pasta. This technology can also be applied to fruit juices and tomato-based products to protect flavor, color and nutritive value over a longer period of time vs. using high barrier package materials (Thomas, 1998).
Addition of enzymes
Other approaches to oxygen scavenging include the addition of enzymes to the surface of films or bottles. Specifically, glucose oxidase and catalase can be added. When water is present, the glucose oxidase will oxidize glucose to gluconic acid and hydrogen peroxide. Then catalase will convert the hydrogen peroxide to water and oxygen. The net result is a reduction in oxygen. These enzymes can be added to the surface of polyethylene or polypropylene (Vermeiren, 1999).
The basic reaction for enzymes to reduce oxygen levels is shown in Figure 3.
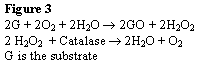
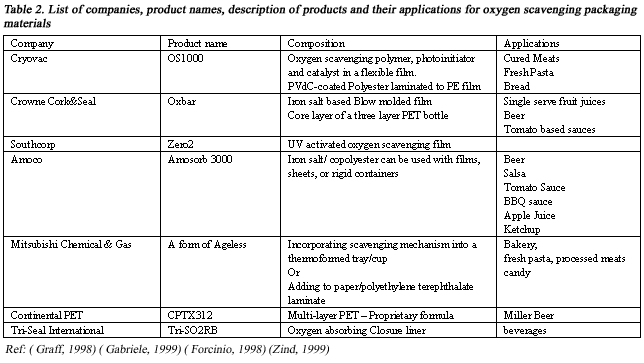
Table 2 lists companies that produce oxygen-scavenging packaging materials.
The benefits of oxygen scavenging
Oxygen scavenger packaging material technology — currently being utilized in beer, fresh pastas, coffee, cured meats, beverages, baked goods and more — offers many benefits to food packaging, including the following:
• Product shelf life is extended considerably.
• There is prevention of aerobic pathogens and spoilage organisms.
• The risk of vitamin oxidation is reduced (i.e. Vitamin A, C, and E).
• The growth of insects and hatching of insect eggs is prevented (i.e. weevils, beetles, etc.).
• The color, flavor and overall freshness of products are maintained.
• There are no additives to the food.
• Markets can be extended for global distribution due to prolonged shelf life.
• Cost savings are realized through reduced waste caused by expired shelf life and less frequently having to replace dated stock.
Sachets were one of the first promising technologies for oxygen scavenging. Since the sachet is an applied packet, there is very little disruption to the package production line. Sachets are also fairly inexpensive in relation to the added value that they provide for the quality of the product. Because sachets are made in a variety in sizes, shapes, absorbency levels and forms, many different foods can be catered towards the specific bio-chemical needs of that food.
Oxygen scavenging film, on the other hand, is a more emerging packaging technology that, as described previously, contains the active material within the film of the package. The benefit to this technology is that the reactants can often be integrated within the various layers of the packaging — adhesive, printing ink, lacquer or enamel as done with cans (Rooney, Chapter 4).
The United States is not as accepting to the idea of oxygen scavenger packaging. Consumers are not in favor of having foreign objects such as sachets in the lining of their product package. With oxygen scavenger film, the consumer cannot physically see the oxygen scavenger activity yet are able to experience its benefit. Currently the drawback to using oxygen scavenger film is that the material is "not generally amenable to economic recycling" (Rooney, Chapter 4). However, the benefits to food safety outweigh the difficulty of recycling.
Potential risks and/or disadvantages
Although there are many benefits to the safety and quality of food products using an oxygen scavenger, there are facts that should be considered when determining its application. One precaution for oxygen scavenger packaging is the prevention of anaerobic pathogen proliferation. Product developers must thoroughly investigate the food system with which they are intending to use oxygen scavengers.
Studies have been done with oxygen scavengers that show pathogens such as Clostridium botulinium Type E surviving in an oxygen scavenger environment (Rooney, Chapter 6). The real risk is that the normal aerobic spoilage organisms that act as indicators for the consumer are not present because of the lack of oxygen in the package. Consumers could potentially eat a product with a pathogen unknowingly if other hurdle technology is not applied to the product.
Another risk in using this method is that some sachets need a certain amount of moisture to activate the oxygen absorption reaction. Lack of moisture present will result in nonactivation of the sachet, thus potentially causing quality issues in the product. There are also some commercial sachets that are very sensitive and could therefore activate prematurely (Gabriele, Michael, p73). If this occurs the sachet must be disposed.
Since the sachet is only exposed to the main area of the package, it is important that the package does not block the path of the absorber. For this reason, sachets are not capable of being used with any liquid foods or any packaging that may have compartments that may entrap oxygen.
There are other concerns that must be considered when working with sachets. One risk is that the sachet has the potential hazard of being consumed. Also, there is the chance that the sachet may leak. Although the reactants within the sachet are not harmful, there may be a problem that could result as product adulteration or product identity (Rooney, Chapter 6).
Conclusion
Though the potential for degradation and adulteration of food products exists, through the use of active packaging and specifically oxygen scavengers, these negative effects are delayed and minimized, which can result in an increased shelf life. Many options are available in the oxygen scavenging arena. Depending upon the application, one might choose from an array of materials and forms ranging from the sachet to the clear lidstock of Cryovac's OS1000.
In addition, more natural options exist as in the case of enzymatic oxygen scavengers. The potential safety and financial benefits that oxygen scavengers lend to a product line make it a viable alternative to traditional and standard packaging applications. Moreover, because it is still considered an emerging technology, much remains to be learned about its potential application in other food industries and systems to further utilize its advantages.
Currently sachets are the main source of oxygen scavenging technology. However, through emerging polymer and film technology, film is moving to the forefront of oxygen scavenging applications.
References
Forcinio, Hallie."Clever Containers," Prepared Foods, March 1998, pp.45-48.
Gabriele, Michael. "Oxygen Scavengers Assume Greater Role In Food Packaging," Modern Plastics, October 1999, p. 73.
Graff, Gordon. "O2 Scavengers Give ‘Smart' Packaging a New Lease on Shelflife," Modern Plastics, February 1998, pp.69-72.
Harima, Y. Food Packaging, Academic Press Publ. London, UK, 1990, pp.229-52.
Nielsen, Tim. "Active Packaging: A Literature Review," The Swedish Institute for Food and Biotechnology, 1997, No. 631.
Rooney, M.L. ed, Active Packaging, Chapters 4-6, Blackie Academic & Professional, New York, 1995.
Thomas, J.; Espinel, K., "Polymeric Oxygen Scavenging System and Its Packaging Applications," presented as a Poster Session at IFT, Annual Meeting June 24,1998.
Vermeiren, L.; Devlieghere, F; van Beest, M; de Kruijf, N; Debevere, J., " Developments in Active Packaging of Foods," Trends in Food Science and Technology 10(1999) pp. 77-86.
Zind, Tom, "Airing Out Oxygen," Food Processing April 1999 pp. 80-81.
About the Authors:
Amanda LaCoste is a graduate from the University of Massachusetts and currently works at Kraft Foods as a Research Scientist in Maxwell House Coffee. She is also attending graduate school at Rutgers University for a Masters in Food Science.
Joanne M. Kaufman is a research scientist, Coffee and Cereal Packaging Research Group, for Kraft Foods in Tarrytown, NY. She has a B.S. in Food Science from Cornell University and is working on a Masters in Food Science from Rutgers University.
Elia R. Shehady is a research scientist at Kraft Foods Inc. in Tarrytown, NY. He has five years experience in product development for Maxwell House Coffee Co. He has a B.S. in Microbiology from Rutgers University and is finishing an M.S. in Food Microbiology from Rutgers.
Keith "Kit" Yam is associate professor of Food Science, Rutgers University, New Brunswick, NJ.
James Schulok has a B.S. in Food Science from Penn State University and is finishing an M.S. in Food Science from Rutgers University. He has three years experience with Specialty Coffee development at Kraft Foods and Maxwell House Coffee Co.
